
Factory Price CE Approved Ctni Troponin I Cardiac Diagnostic Test Kit (whole blood/serum/plasma)
Description
Basic Info
| Model NO. | test strip/cassette |
| Specification | 25T/box; 40T/box |
| Trademark | CLUNGENE |
| Origin | China |
| HS Code | 382219 |
| Production Capacity | 5000000000/Year |
Product Description
Troponin I/Ck-MB /MYO Rapid Test Cassette with CE Approved Cardiac Marker Test Ctni Test Kit
CLUNGENE Cardiac Troponin I Rapid Test Cassette (WB/S/P) is a lateral flow immunoassay for the qualitative detection of cardiac Troponin I (cTnI) and its complex in human whole blood, serum or plasma.
Product Features:
1. Easy handling, no instrument required.2. Results at 15 minutes.3. Results are clearly visible and reliable.4. High Accuracy.5. Room temperature storage.
Product Information
| Catalog No. | Specifications | Format | Specimen | Reading Time | Storage Condition | Shelf Life | Certification |
|---|---|---|---|---|---|---|---|
| CTNI4002 | 40 tests/box | Cassette | Whole Blood,serum, plasma | 15 minutes | 4-30ºC | 24 months | CE |
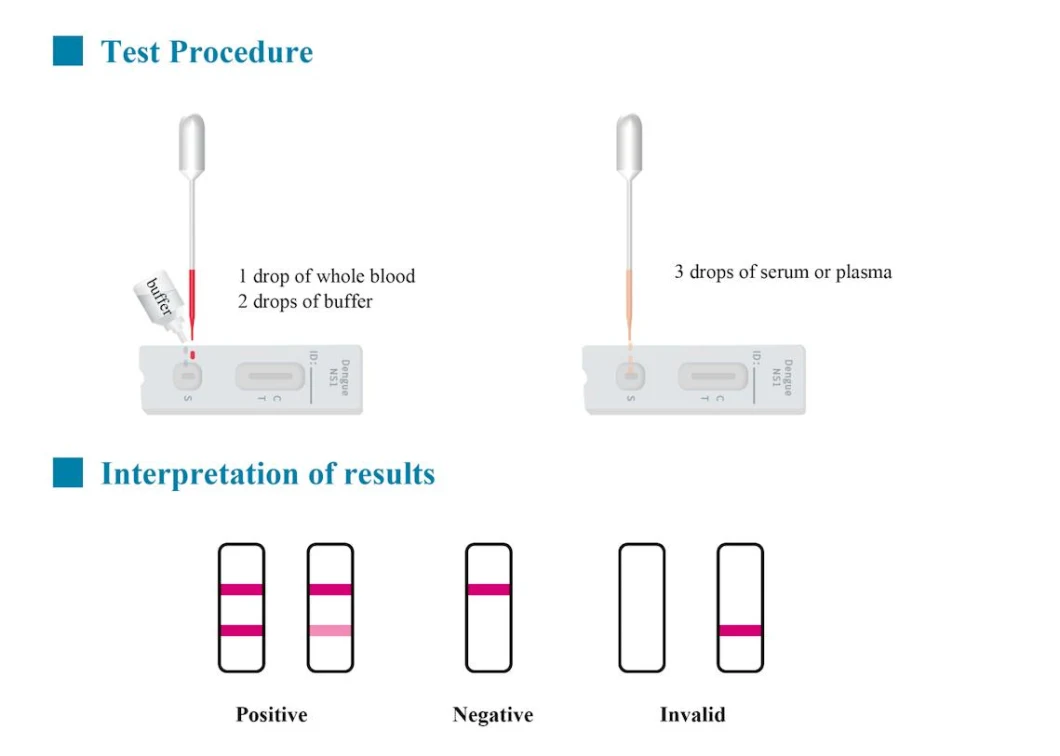
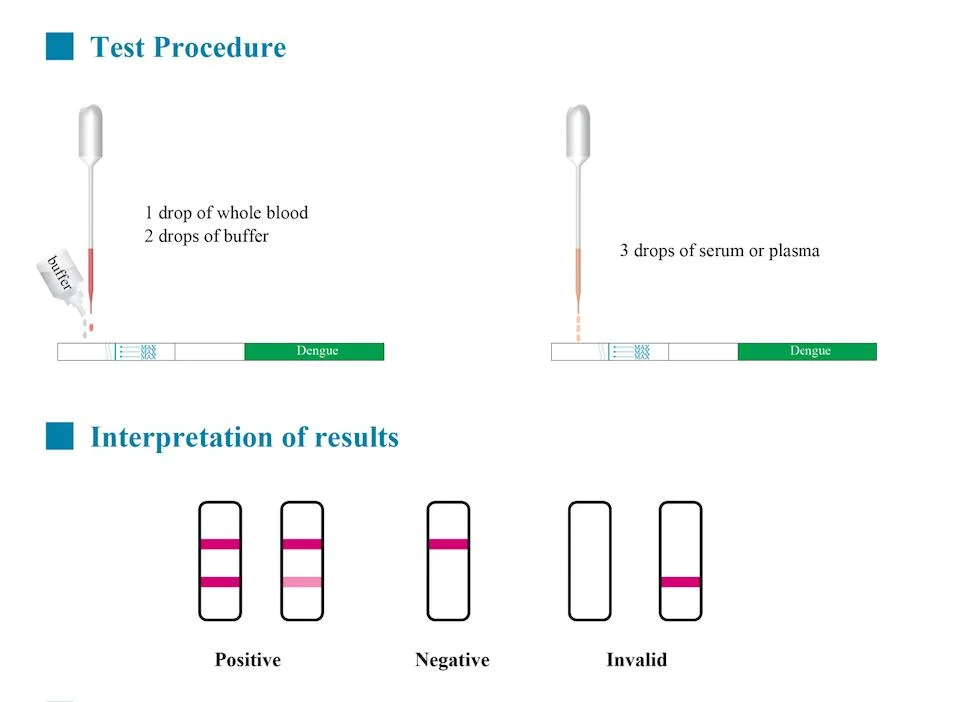
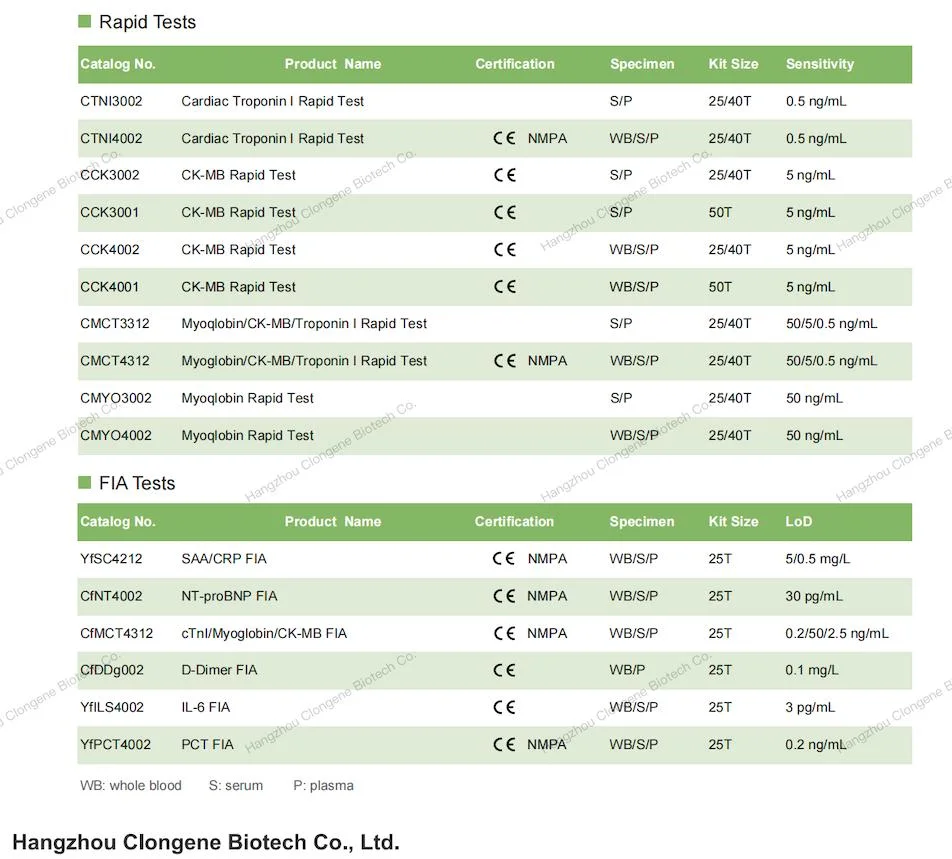
Company Profile

Hangzhou Clongene Biotech is a national high-tech enterprise based in Hangzhou, China. We are specializing in the R&D, production and sales of biological raw materials, IVD reagents and instruments. In addition, we can also provide CRO & CDMO services. As a well-known supplier of biological raw materials in China, we provide products and services to major domestic IVD products manufactures and scientific research institutions. Our main IVD reagents have obtained authoritative certifications including NMPA of China, FDA 510(K) clearances of US, TGA of Australia, CE of EU and some other certifications from different countries. The products have been sold well in more than 100 countries and regions around the world. We can also provide customers with high-quality technical services, including protein expression services, recombinant antibody expression services and CHO-K1 stable cell line construction services.
While continuously innovating the products, we always commit to product innovation and strict quality control. We pursue to provide our customers with high-quality products and excellent service through infinite trust and responsibility.
Production Line
Quality Control
Research & Development
Certifications & Glories
Prev: Tp-Wms-Il Outdoor Weather Station
Next: Ctni Cardiac Troponin I Immunofluorescence Poct Reagents Kits
Our Contact






