
Colloidal Gold Method Rapid Infectious Diseases Test Kit Swan Test Kit for 25 Person
Product Overview Model: BF/YFSpecification: 25T/KitBox size: 18.0x14.0x10.5cmCarton size: 730x387x468mm 1000T/cartonAdva
Description
Basic Info
| Model NO. | BF/YF |
| Type | IVD Instrument |
| Model No. | Bf/Yf |
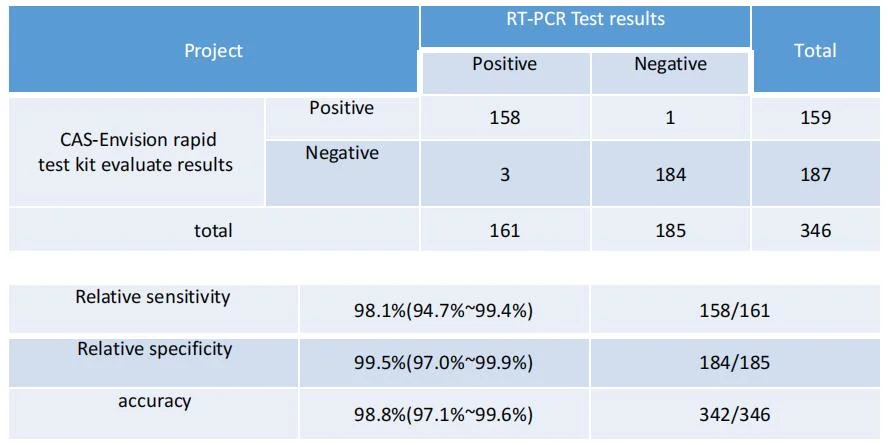
| Accuracy | 98.4% |
| Sensitivity | 97.5% |
| Specificity | 99.2% |
| Certificate | CE/ISO13485/9001/FSC/White List |
| Sampling Methods | Nasal/Pharyngeal/Saliva Swabs Are Available |
| Shelf Life | 24 Months |
| OEM/ODM | Support |
| Operation Temperature | Room Temperature |
| Methodolgy | Colloidal Gold |
| After-Sale Service | Return and Replacement |
| Fomat | Cassette |
| Packaging | 25 Test/ Kit |
| Application | Test Center, Home Using |
| Transport Package | Box+Carton |
| Specification | disposable |
| Trademark | CAS-Envision |
| Origin | China |
| HS Code | 3822009000 |
| Production Capacity | 5000 PCS/Day |
Product Description
Product OverviewModel: BF/YFSpecification: 25T/KitBox size: 18.0x14.0x10.5cmCarton size: 730x387x468mm 1000T/cartonAdvantage: Fully enclosed extraction solution, maintain stable performance.
| Components | Sample | Storage | Style |
| 25 Individual foil bags, each contains:• 1 Test cartridge • 1 Desiccant pouch25 Sample extraction tubes;25 Sampling swabs;25 Packs of extraction solution;1 Instructions for use;1 Operation flow card;1 QC card. | Nasopharyngeal or oropharyngeal swab | 2~30ºC | Test cartridge |

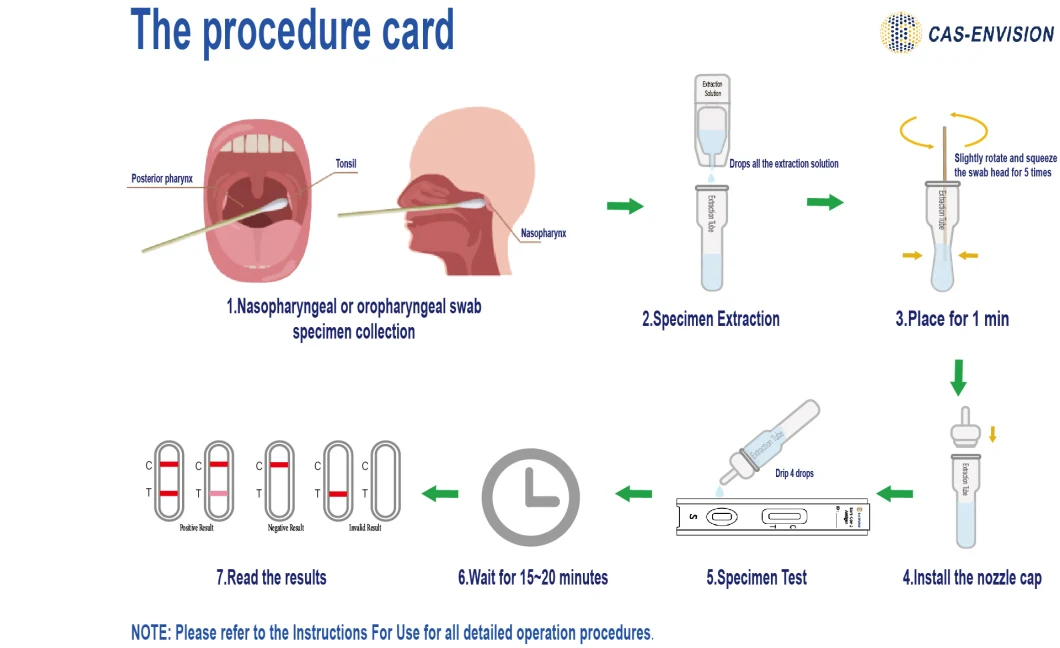
Product Actual Photo


In contrast, antigen testing can detect the virus during the incubation period,the acute phase, or the early stages of the disease. It is more suitable for early diversion and rapid management of large-scale suspected populations.
Advantagas of Antigen Rapid Test
1. High sensitivity and specificity;
2. The pathogen can be detected at the initial stage of infection;
3. Easy sample collection and simple operation;
4. No professional technicians and equipment are required;
5. Quick results in 15 minutes;
6. Available for home or professional use.
Sensitivity and Specificity: The nasal swabs of 161 patients with nucleic acid positive and 185 subjects with nucleic acid negative were tested simultaneously using the product and PCR method to evaluate the sensitivity, specificity and accuracy of the product; The NP swabs test results were compared with PCR, The sensitivity, specificity and accuracy were calculated and the 95% confidence intervals were calculated resp ectively.
Qualification certifications our products have obtained
The CAS-Envision Antigen Rapid Detection Kit have passed the qualification certification of EU CE, Thailand, Malaysia, Czech Republic, Australia, France, Brunei, German (BfArM+PEI), Zimbabweand.In additon, it has also passed the ISO13485, Whitelist of the Ministry of Commerce of China, the EU Common List and other certifications.
CE CertificationPaul-Ehrlich-Institut
Manufacturer Introduction
Shenzhen CAS-Envision Medical Technology Co. LTD. is a high-tech start-up originated from Shenzhen Institutes of Advanced Technology (SIAT) and Chinese Academy of Sciences (CAS). The team came from the "artificial retina" Guangdong Innovation Team and Shenzhen Peacock Team which attracted many oversea talents. And the R&D fee over 100 million RMB has been financially supported by local governments and Chinese Academy of Sciences.
Manufacturing Package Real Photos In Stocks
CAS-Envision Corporate Headquarters
At this critical moment, CAS-Envision has upheld the initial intention to benefit mankind with science and technology. We combined our advantages of the medical sterilization experiences and the high-standard cleanroom, together with the rapid integration of resources, and achieved the mass production of various medical goggles, medical protective clothing, etc. We are now committed to the professional total solution for personal epidemic protection, and provide epidemic prevention products including medical goggles, medical face shields, medical protective clothing, gloves, face masks.
We are doing our best to provide critical products for the global effort on epidemic prevention.
FAQ
Q: Are you a trading company or manufacturer?
A: We are a manufacturer of medical consumables, focusing on the development and manufacturing of diagnostic equipment.
Q: How long is your delivery time?
A: Usually it takes about 2-10 days after the deposit is received, it depends on the quantity
Q: Is free sample available?
A: Yes, we are glad to offer you some samples for free, but freight shall be collected at your side
Q: How to get a quotation?
A: Please tell us the products and the quantity you need, we will offer you our best prices
Q: Can you design for us?
A: Of course, we accept various OEM and ODM orders
Q: What is the payment way?
A: TT, Paypal.
Q: What other products do you have?
A: Besides Antigen rapid test kits, we also supply medical safety goggles, conjoined medical protective clothing, disposable non-woven face mask, face shield and and other personal epidemic prevention products. And we also support exporting materials if needed.
Contact me for Quotation, Complete Certifications, Instruction Manual and Clinic Report!
Rachel Yang Overseas Sales Manager4F, B 4, Tusincere Technology Park, No. 333 Longfei Avenue, Longcheng Street, Longgang District,Shenzhen,Guangdong China.
Prev: One Step Rapid Test Kit Medical Healthcare Infectious Diseases Test HCV/Hbsag/HIV
Next: CAS 79804-71-0 Crf (OVINE) Trifluoroacetate / Corticotropin-Releasing Hormone (ovine)
Our Contact
Send now






