
Aiv H9 AG Test Kit Avian Influenza Virus H9 Antigen Rapid Test
Description
Basic Info
| Model NO. | 108916 |
| Specimen | Secretions |
| OEM/ODM | Available |
| Box Specification | 20 Tests/Box |
| Certificate | ISO 13485 & ISO 9001 |
| Quantity Per Carton | 880 Tests |
| Weight/Carton | 13.50kg |
| Shelf Life | 24 Months |
| Test Time | 5-10 Minutes |
| Transport Package | 64*36*36.5cm |
| Specification | 155*150*68mm |
| Trademark | Testsealabs |
| Origin | China |
| HS Code | 3005109000 |
| Production Capacity | 3 000 000 Tests/Day |
Product Description
| Format | Cassette |
| Specimen | Secretions |
| Box Specification | 20 Tests/Box |
| Certificate | ISO 13485 & ISO 9001 |
| Shelf Life | 24 Months |
| Box Size | 155*150*68mm |
| Carton Size | 64*36*36.5cm |
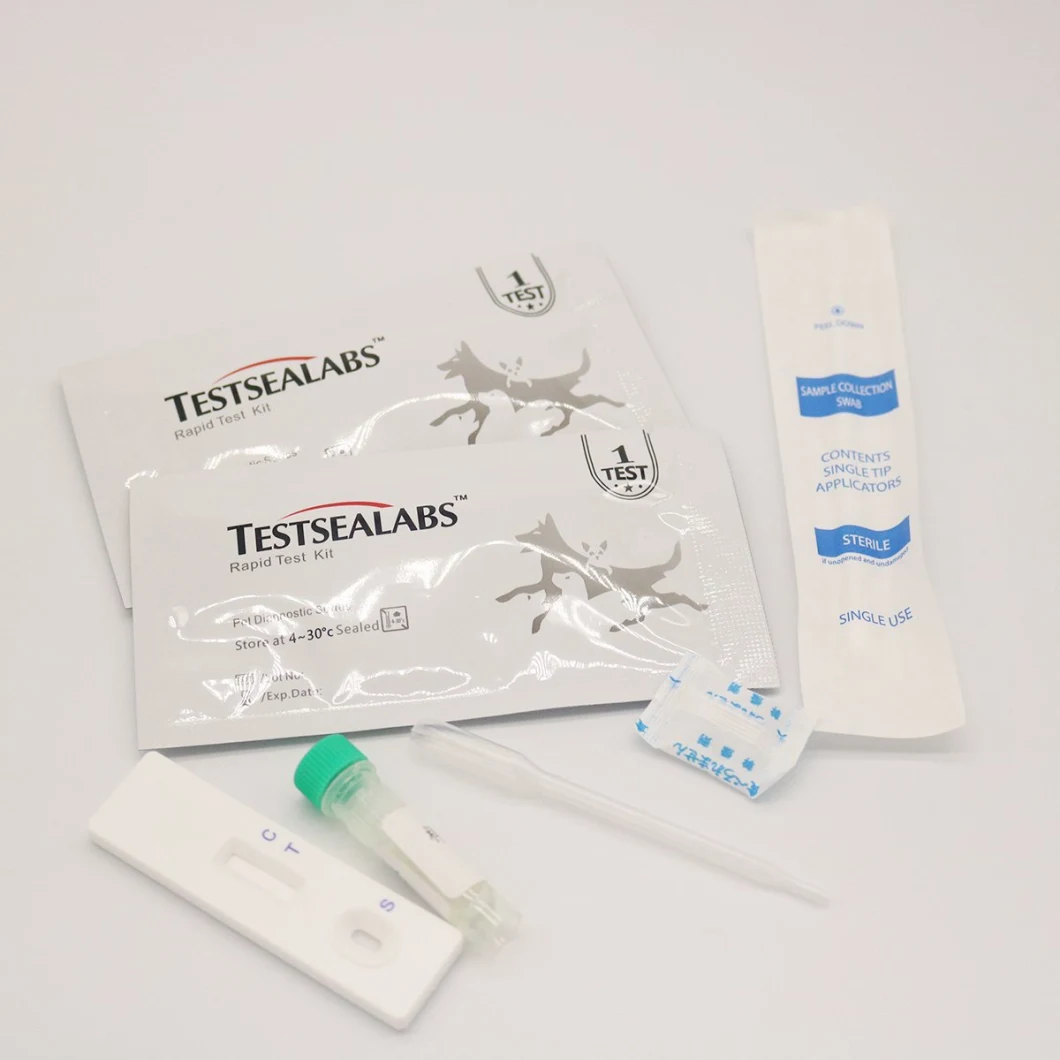
Detailed Photos


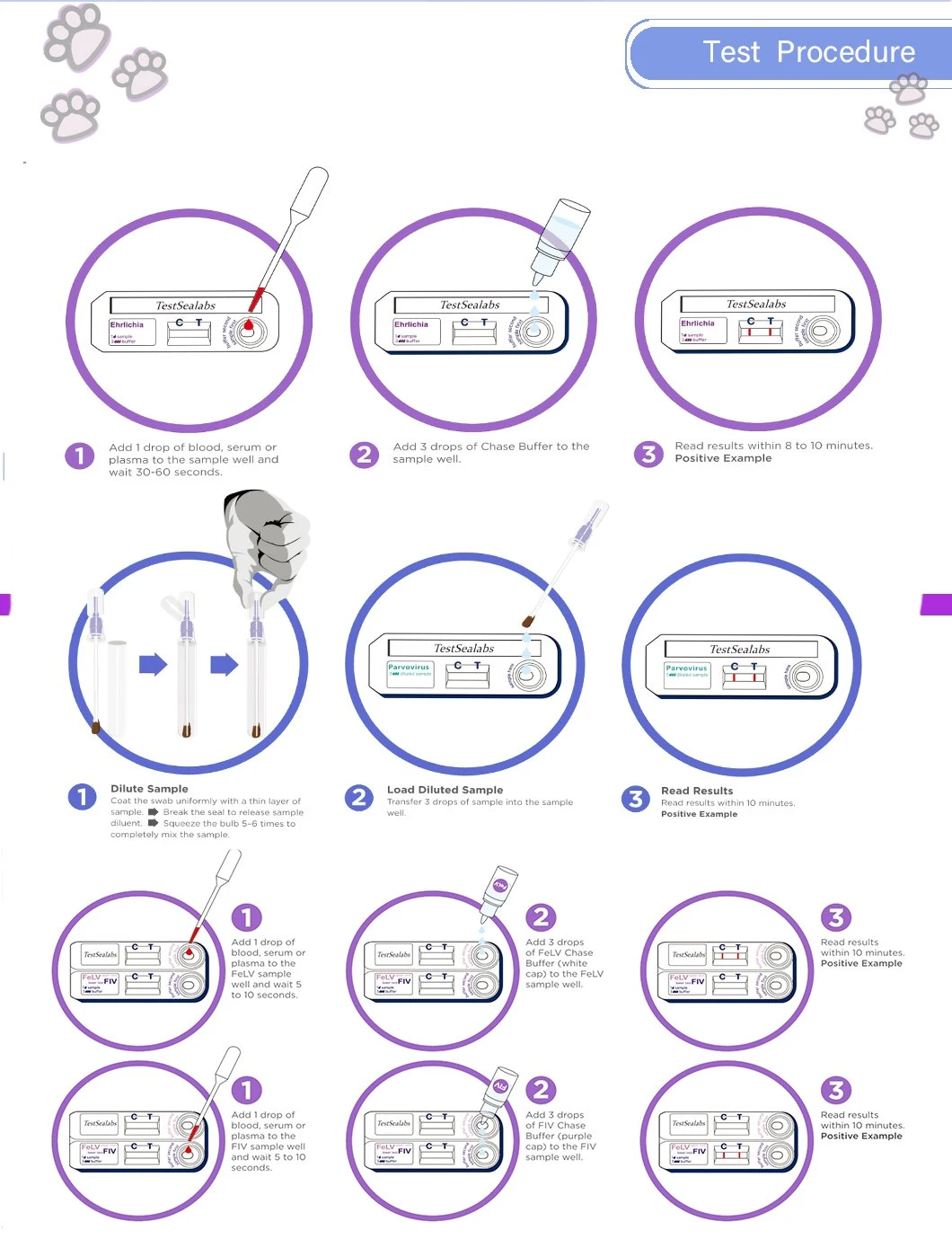
Test Procedure
Product List
Company Profile
Hangzhou Testsea Biotechnology Co., LTD. is a national high-tech enterprise, is located in Hangzhou. Testsea has lots of researchers and workers who graduated from Zhejiang University and overseas. Testsea is specialized in the research, development, production and sales of raw materials for medical diagnosis and food safety testing. We have applied for 28 kinds of patents which cover medical diagnosis, food safety rapid testing, food enzymatic immunoassay and new enzyme preparation. Testsea provides perfect raw material solutions for scientific research institutions, enterprises, research institutes and other institutions globally. Testsea total business area is more than 56,000 square meters, including 2,000 square meters of GMP 100,000-level purification workshop, our company is strictly following ISO13485 and ISO9001 quality management system operation with research, production, quality control, finance, domestic sales and international sales, etc. Our products are well accepted by many users domestic and abroad. In addition, we establish a good business relationship with many domestic universities and in vitro diagnostic production enterprises, even with southeast Asia, Europe, Africa, Latin America, and other countries.Testsea has a research and development team led by doctors and masters with professional workers and a well-equipment facility. The production capacity of recombinant antigens has reached 18g/month.Testsea pursuit of "integrity, quality, responsibility" concept and adhere to the quality, the purpose of serving the society and constantly developing new high-quality diagnostic materials efforts.
Certifications
Our Contact






