
Ctni Test Ctni Rapid Test Kit Rapid Diagnostic Test Kit
Overview Product Description EVANCARE Diagnostic Cardiac Troponin I Rapid Test Kits For professional and in vitro diagno
Description
Basic Info
| Model NO. | EC-CTNI |
| Function | Early Detection |
| Display | Screening |
| Format | Strip+Bottle |
| Name | Ctni Test Kit |
| Equipment | by Eye or Machine |
| Reaction Time | 5-10 Seconds |
| Accuracy | 99.7% |
| Delivery Time | 3-5 Days |
| Temperature | 4-30 Degree |
| Storage | Room Temperature |
| Transport Package | Carton |
| Specification | 64*50*44 cm |
| Trademark | evancare or OEM |
| Origin | China |
| HS Code | 3002150090 |
| Production Capacity | 100000PCS/Day |
Product Description
Product Description
EVANCARE Diagnostic Cardiac Troponin I Rapid Test KitsFor professional and in vitro diagnostic use only.
[INTENDED USE]The Cardiac Troponin I Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of cardiac Tropnin I (cTnI) and its complex in human whole blood, serum or plasma. It provides an aid in the diagnosis of acute myocardial infarction (AMI). [PRINCIPLE] Cardiac Troponin I (cTnI) is a cardiac muscle protein with a molecular weight of 22.5kilodaltons. Together with troponin T (TnT) and troponin C (TnC), TnI forms a troponincomplex in the heart to play a fundamental role in the transmission of intracellularcalcium signal actin-myosin interaction. The human cTnI has additional amino acid residues inits N- terminal that does not exist in the skeletal forms thus making cTnI a specific cardiac marker. Normally the level of cTnI in the blood is very low. cTnI is released into the bloodstream in forms of free cTnI and cTnI-C-T complex at 4-6 hours after myocardial cell damage. The elevated level of cTnI could be as high as 50ng/ml during 60-80 hours after AMI and remains detectable for up to 10 -14 days post AMI. Therefore, circulating cTnI is a specific and sensitive marker for AMI. The Cardiac Troponin I Rapid Test is a simple, visual qualitative test that detects cardiac Tropnin I in human whole blood, serum or plasma. It is a noninvasive method and does not use radioactive isotopes. The test is easy to perform and requires no specialized equipment. Visual interpretation provides an accurate qualitative result. It is a useful on-site aid in the diagnosis of acute myocardial infarction (AMI). .
Detailed Photos
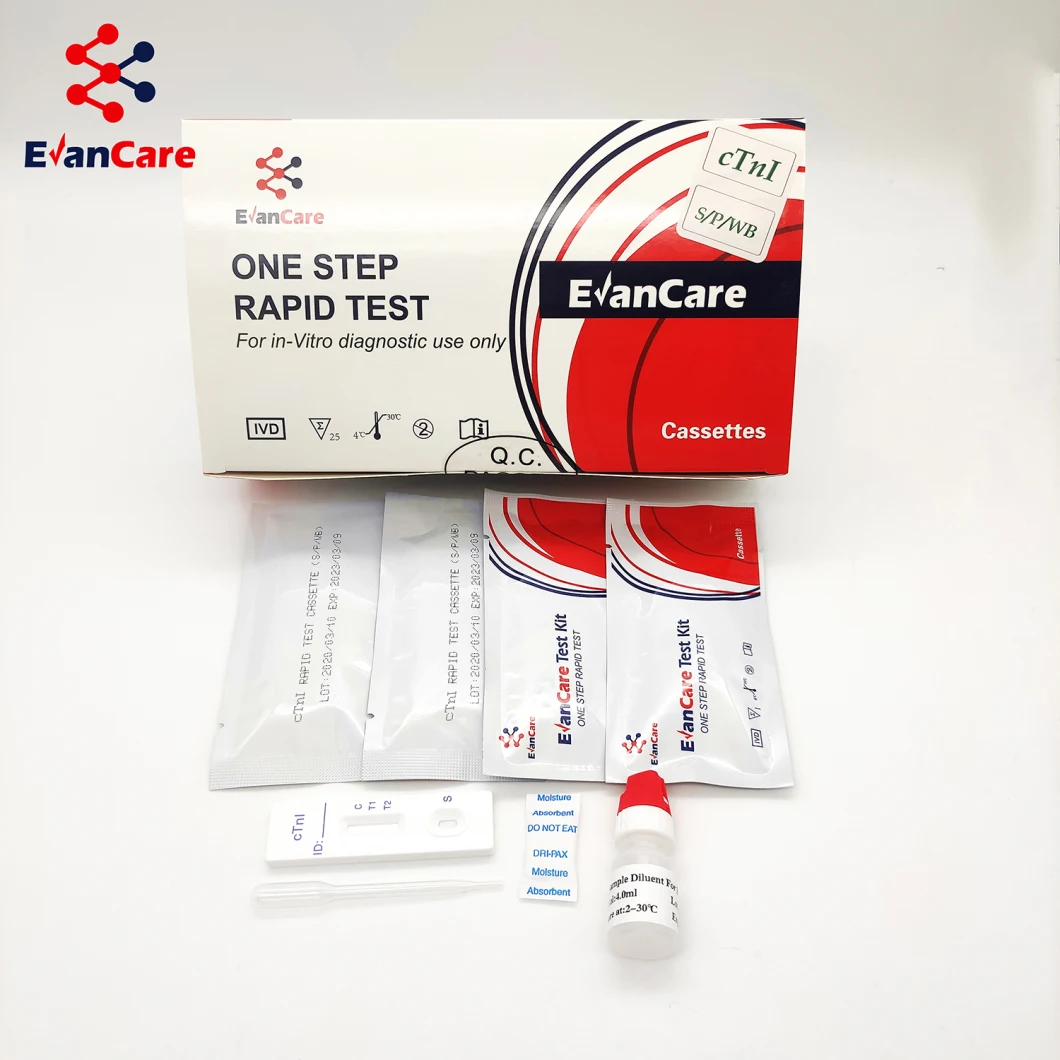
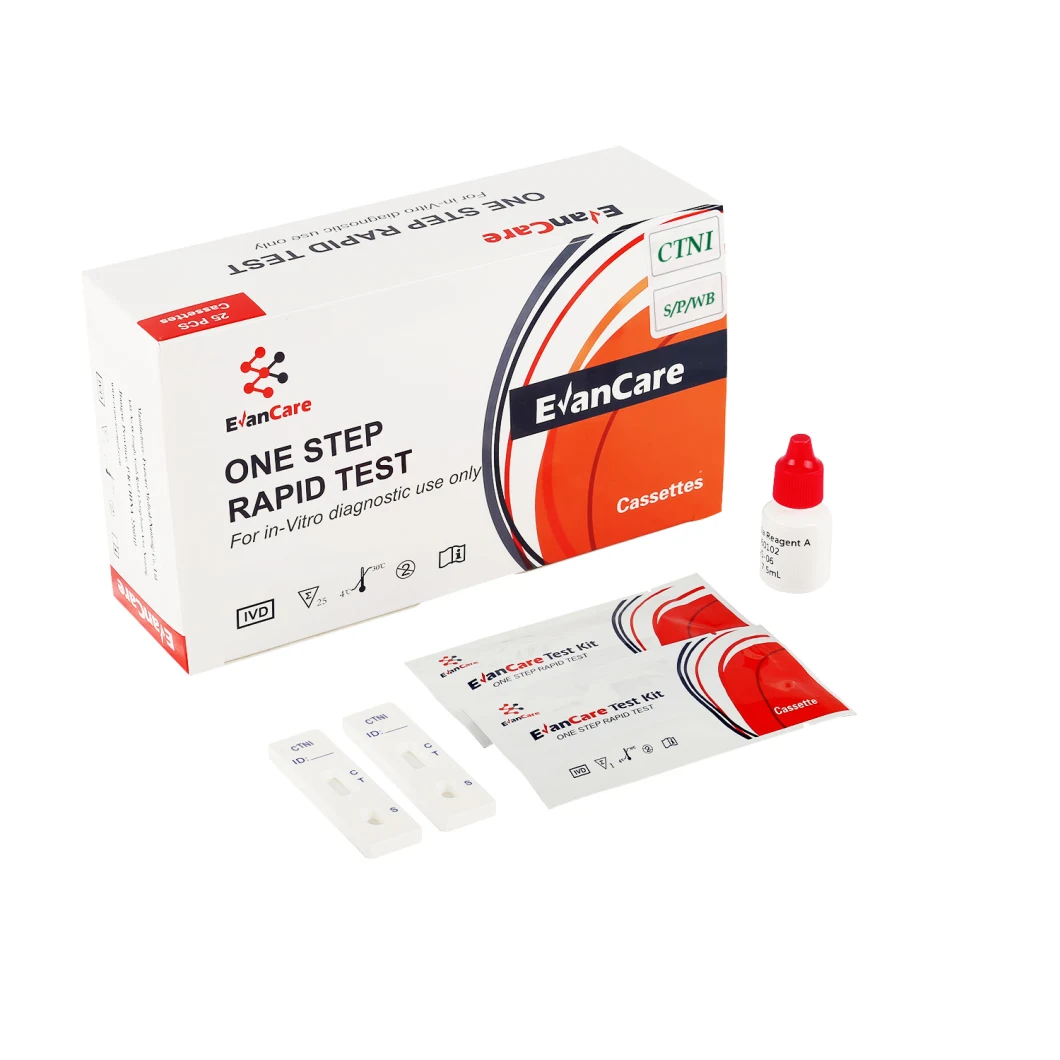
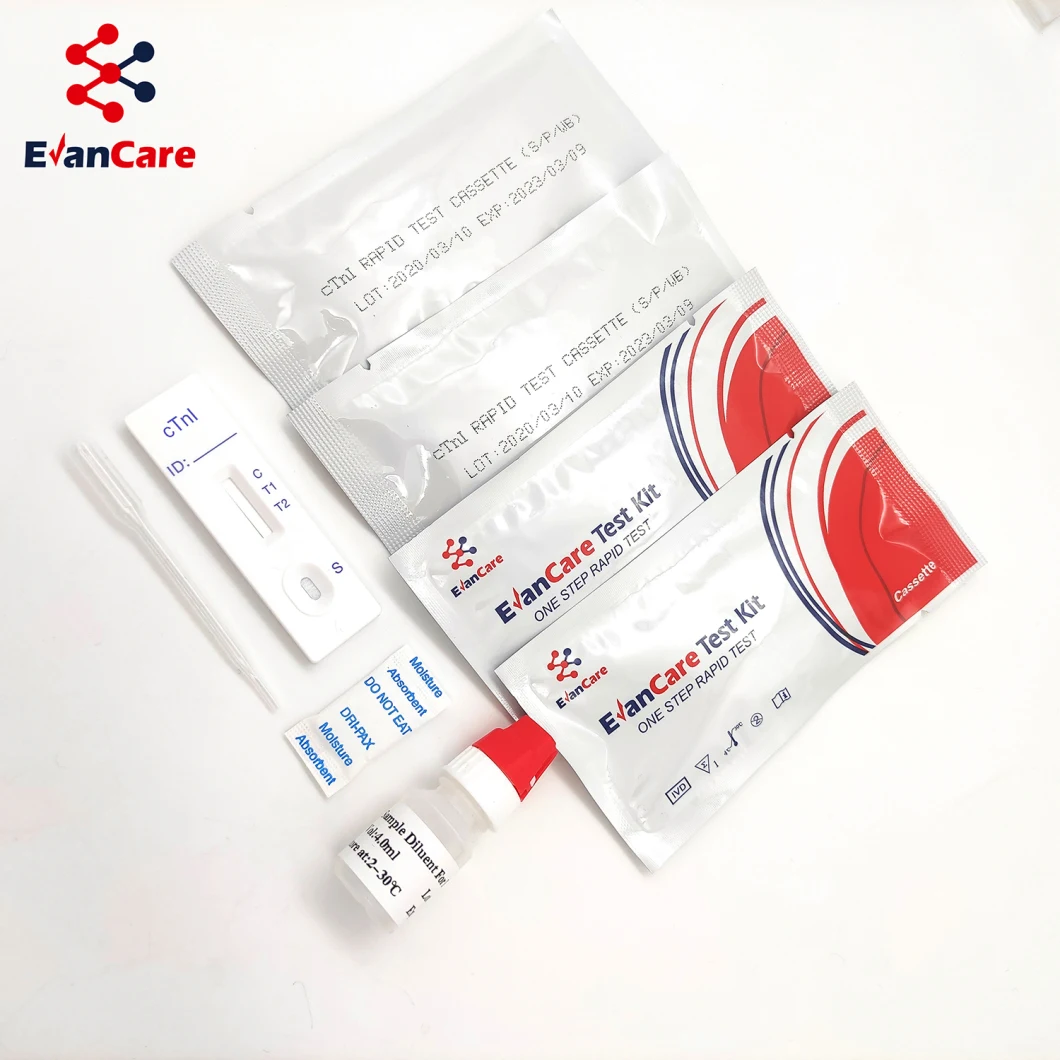
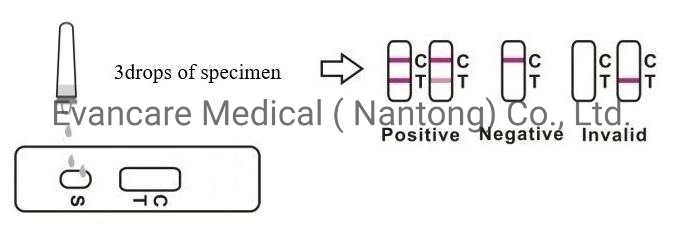
Positive: *Two lines appear. One colored line should be in the control region (C), and another apparent colored line adjacent should be in the test region (T). Negative: One colored line appears in the control region (C). No line appears in the test region (T). Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test cassette. If the problem persists, discontinue using the lot immediately and contact your local distributor.Item No. | EVANCARE Diagnostic Cardiac Troponin I Rapid Test Kits |
Contents | Each bag:1 test cassette and 1 desiccant(cassette) 25 cassettes+1 Buffer /box antigen test We can put instruction as your requirement |
Format / Size | Cassette:2.5mm 3.0mm 4.0mm |
Methodology | Sandwich Colloidal Gold |
Assay Type | Qualitative Detection |
Specimen | Antibody (S/P/WB), |
Indicator | Cardiac Troponin I Test Cassette |
Storage Temperature | 4-30 degree centigrade |
Expiry date | 3 years |
Certificate | CE,ISO,FREE SALE,FDA... |
Detection Limits | Home or Professional Use |
Accuracy | > 99.99% |
| Reaction Time | 3-5 min |
| Delivery | 5-7 days |
| OEM Service | Available |
| Shipping Method | by air or sea or international express |
Company Profile
Certifications
Product Procedure
Activity Exhibition

FAQ
Our Contact
Send now






