
CE Approved Medical Disposable Infectious Disease Virus Detection Kit Cassette
Description
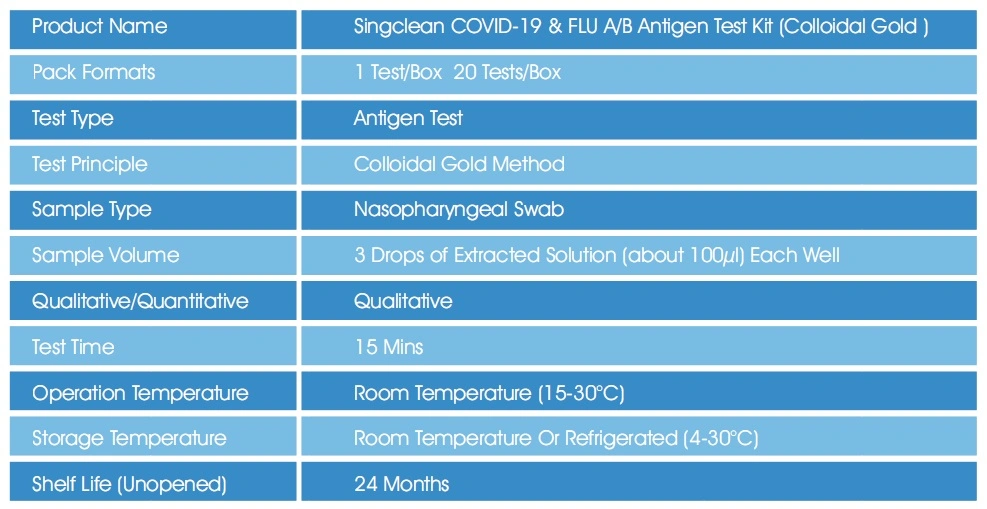
Basic Info
| Accuracy | 97% |
| Transport Package | 500test/Carton; 600test/Carton |
| Specification | 1 test/box; 20 test/box; 50 test/box |
| Trademark | Singclean |
| Origin | China, Hangzhou |
| HS Code | 3002150090 |
| Production Capacity | 50000/Day |
Product Description
CE Approved Medical Disposable Infectious Disease Virus Detection Kit Cassette
Product Description
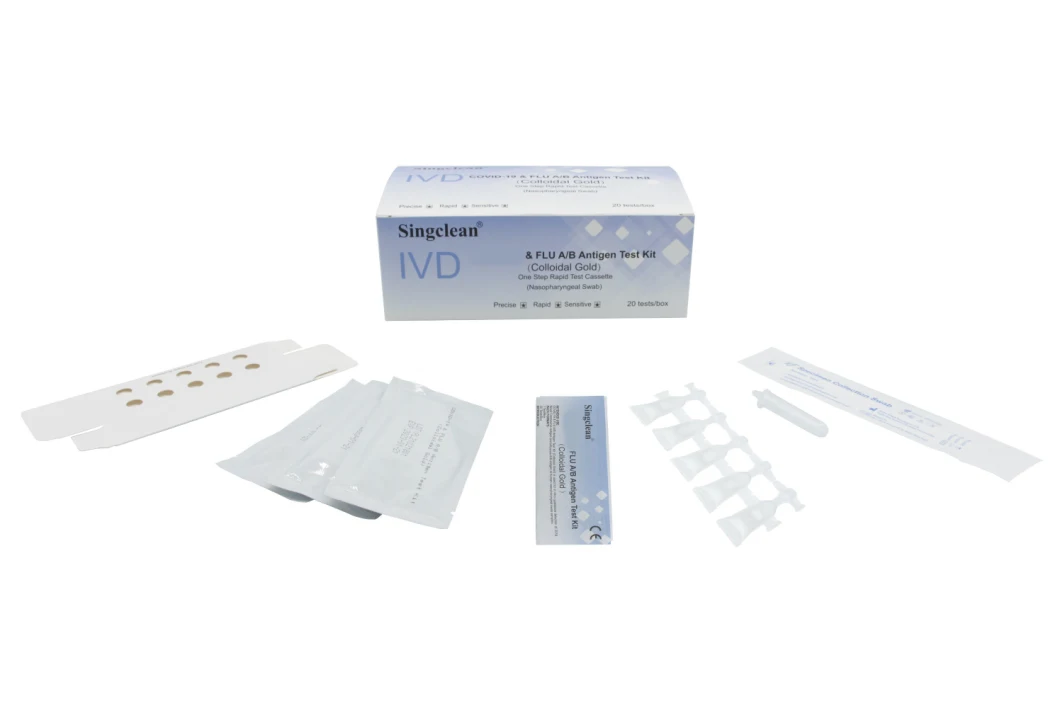
Singclean C-19 & FLU A/B Test Kit (Colloidal Gold) is used for in vitro qualitative detection of 2019 Novel virus and influenza A/B in human nasopharyngeal swab samples.
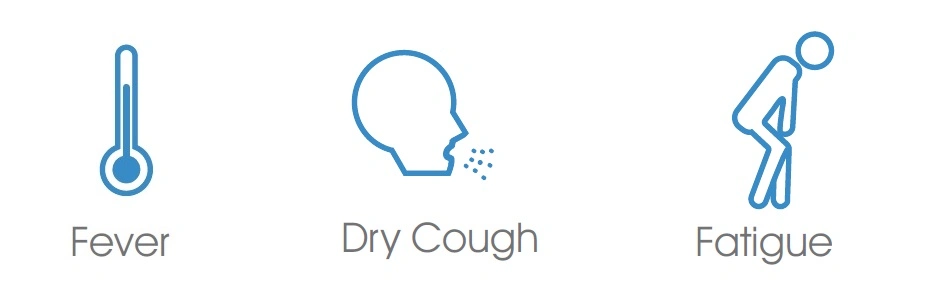
This test provides only a preliminary test result. Therefore, any reactive specimen with the test kit (Colloidal Gold Method) must be confirmed with alternative testing methods and clinical findings.IntroductionBoth C-19 and influenza A/B viruses(Flu A/B) can cause acute respiratory infections, and people are generally susceptible. C-19 and Influenza A+B are generally detectable in upper respiratory samples during the acute phase of infection. These three vireses are highly contagious, spread quickly, have a short incubation period, and have a high incidence. Symptons of C-19 and influenza may look similar, the main Symptions includeWith this combination test, healthcare workers can provide an accurate diagnosis to patients and treat them with the appropriate measures.Features
1.Results ready in 15minutes2.Accurate diagnostic tool for active infection3.Easy to administer and read results4.Affordable, no need for instrument, highly portable5.Enable testing on a massive scale6.For healthcare workers use only
Product Photos
Product informationClinical DataStorage and StabilityThe test kit can be stored at room temperature or refrigerated (4-30oC). The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.Do not freezeDo not use beyound the expiration dateDo not store the test kit in direct sunlightAfter opening the sealed pouch, use the test as soon as possible within 60 minutes.
| No. | Product Name | Specimen | Format | Qty/Kit | Regulatory Status |
| 1 | Singclean C-19 IgG/IgM Test Kit(Colloidal Gold Method)- Antibody | Whole Blood/ Serum/ Plasma | Cassette | 1test/box, 20test/box | CE |
| 2 | Singclean C-19 Test Kit(Colloidal Gold Method) | Nasopharyngeal Swab | Cassette | 1test/box, 20test/box | CE |
| 3 | Singclean FLU A/B Test Kit(Colloidal Gold Method) | Nasopharyngeal Swab | Cassette | 20test/box | CE |
| 4 | Singclean C-19 & FLU A/B Test Kit(Colloidal Gold Method) | Nasopharyngeal Swab | Cassette | 20test/box | CE |
Main Products
Company Profile
Prev: One Step Rapid Infectious Disease Syphilis Test Kit with High Accuracy Syphilis Test
Next: Infectious Disease Antigen AG Rapid Testing Kits Shenzhen Supplier Ready Ship in Stock
Our Contact






