Antigen & Influenza a/B Rapid Test Kit High Quality Factory Supply Infectious Disease Diagnostic
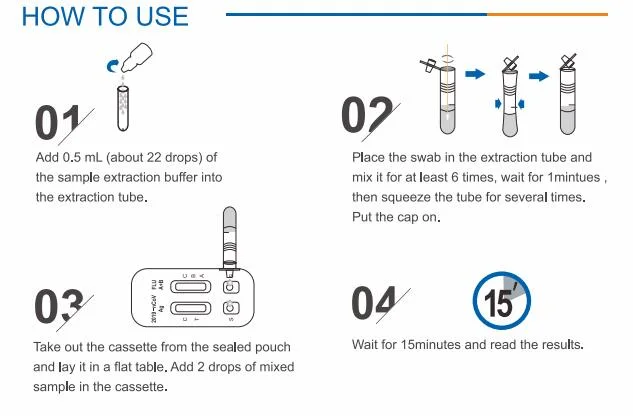
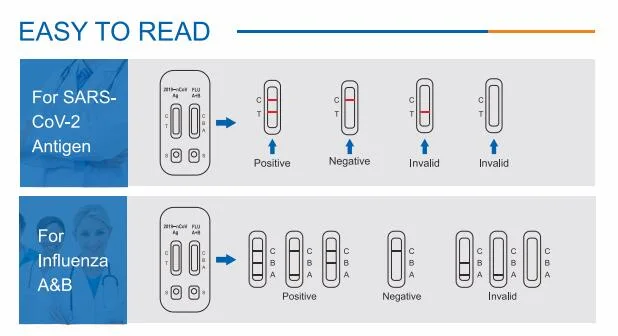
Antigen & Influenza A/B Rapid Test Kit (Immunochromatography)INTENDED USEInfluenza A/B Rapid Test cassette provides a si
Description
Basic Info
| Operation Temperature | Room Temperature |
| Transport Package | Carton |
| Specification | 20 Test/Box |
| Trademark | LABNOVATION |
| Origin | China |
| HS Code | 3002150050 |
| Production Capacity | 500000/Day |
Product Description
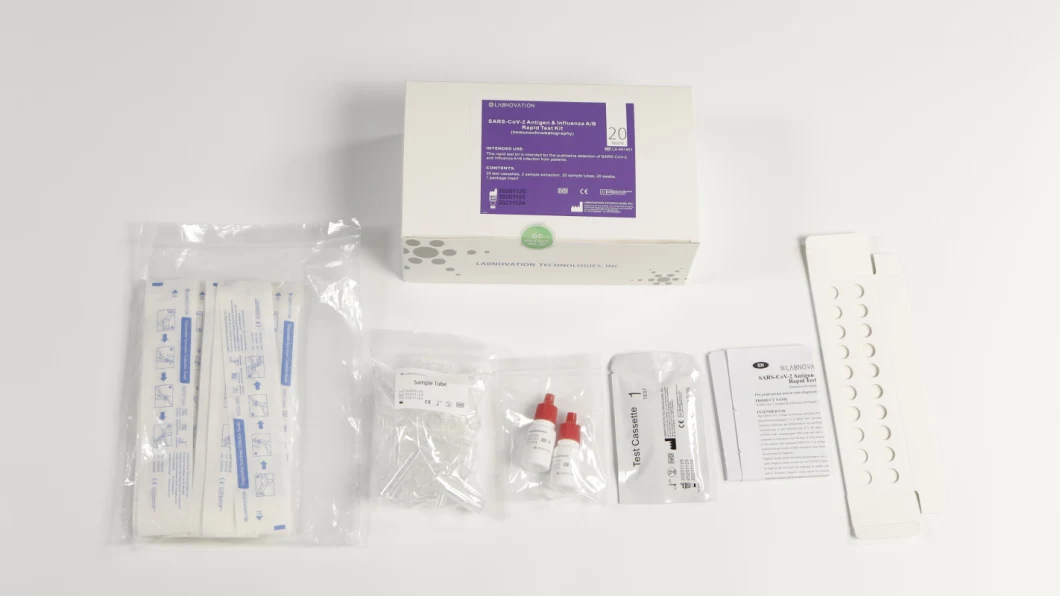
Antigen & Influenza A/B Rapid Test Kit(Immunochromatography)INTENDED USEInfluenza A/B Rapid Test cassette provides a simple,rapid detection as an aid in the differential diagnosis of influenza A & B using nasal swab specimens. This easy-to-use product and rapid results can be used for the screening of early infected patients and asymptomatic patients. It is also effective supplement for nucleic acid dection.KIT COMPONENTS
Test cassettes,Sample extraction, Sample tubes, Swabs, Instruction Manual
Diagnostic Accuracy
| Item | Antigen test strip performance against PCR | Influenza A test strip performance against PCR | Influenza B test strip performance against PCR |
| Sensitivity | 96.30% | 93.20% | 87.20% |
| Specificity | 97.30% | 96.20% | 97.90% |
| Global high frequency mutation | Alpha / B.1.1.7 (U.K.) | Beta / B.1.351 ( South Africa ) |
| Gemma / P.1 ( Brazil ) | Kappa / B.1.617.1 ( India) | Delta / B.1.617.2 ( India) |
| C.37,etc. | Alpha / B.1.1.7 ( U.K.) | B.1.36.16.etc. |
| A.2.5, etc. | A.23.1 | Alpha / B.1.1.7 ( U.K.) |
| Lambda / C.37(Peru) | B.1.1.33.etc. | C.1.1. etc. |
CERTIFICATE
Company Information
LABNOVATION TECHNOLOGOES, INC was established in 2001 and has been recognized as a "National High-tech Enterprise". The registered capital (including its wholly-owned subsidiaries) is RMB 20 million, and it is one of the earliest companies exporting in vitro diagnostic reagents in China. The product sales network has covered more than 110 countries and regions around the world.It is the first in the industry to pass the ISO9001:2008.ISO13485:2012/AC:2012 quality system TUV certification, and obtain the EU CE certification and FDA certification.The company invests more than 10% of its sales into product research and development, hardware construction and update every year, and 85% of the company's R&D team employees have more than 5 years of industry experience.

Prev: Fertility and Infectious Disease Disposable Medical Supplies HCG Pregnancy Rapid Test Cassette
Next: One Step Rapid Infectious Disease Syphilis Test Kit with High Accuracy Syphilis Test
Our Contact
Send now






